-

Vertical Ridge Augmentation
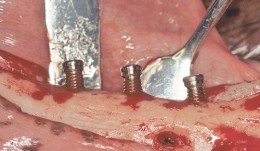
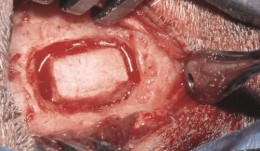
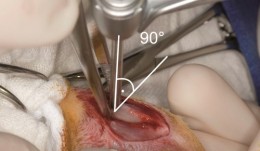
Vertical augmentation is essential for restoring bone defects in patients with destructive gum disease, before rigid fixation of implants or grafts. However, in practice it is associated with high complication rates and limited success. Thus it is important to refine the techniques used by dental surgeons through preclinical research. This chapter looks at various animal studies, and focuses on one specific validated, reproducible and reliable protocol. This involves creating a saddle-like, bony, critical-size defect in the mandible of dogs, in which sufficient time is allowed for the defect to become chronic, thus mimicking bone atrophy in humans. Vertical bone gain is assessed following placement of a tissue-engineered block. Surgical and flap management techniques can be tested, as well as different biologics, devices, scaffolds, membranes, implants and screws. Assessment relates to the initial defect, as well as responses over time relating to resorption of graft particles, soft tissue swelling and inflammation and bone destruction and formation. Osseointegration is quantified to indicate success or failure of the intervention. The protocol can also be used to compare analytical methods. The methods have fairly predictable outcomes, and are applicable to patients with traumatic tooth extraction, jaw damage, endodontic infections and failed implants. -

Sinus Floor Augmentation
The maxillary sinuses are located inside the cheekbones, above the upper jaw, from the second premolar area to the wisdom teeth. In some people with tooth loss, the sinuses are too close to the upper jaw for dental implants to be placed; in others, bone may have been reabsorbed because of gum disease. In either case, bone height can be restored using a sinus lift – an increasingly common technique in dental practice. A small hole is made in the bone beneath the gum and the membrane lining the sinus is pushed away from the jaw to create a space into which bone graft material can be packed. Implants can then be placed after the graft has integrated with the natural tissue. Preclinical models are necessary for testing bone grafts from different sources, and different bone substitution materials, biologically active coatings, growth factors, and implant types. The vascularization process can also be investigated. This chapter discusses the merits of various animals for studying the pathology and repair of sinus defects, with a preference for those with similar sinus structures, bone loading characteristics and remodeling processes to humans. Osseointegration of graft materials can take many months, so the protocols here provide a framework of suitable timings and sequences of surgical procedures, including the placing of implants at same time as augmentation. Suitable endpoints are defined too to assist investigators in planning and reporting on reproducible, relevant outcomes. -


Peri-implantitis Defect
Inserting implants immediately after tooth extraction reduces clinic appointments, but some patients experience breakdown of their gum and bone tissues; this early exposure of the implant inhibits bone regeneration and implant success. Increasing numbers of people have implants nowadays, more of which contain titanium, which is good for osseointegration, but tends to harbor bacteria if the surrounding bone shrinks away. Preclinical research is therefore important for investigating the nature of implantitis and its relations to bacterial plaque formation. Flap procedures need to be optimized to ensure adequate wound closure and prevent wound breakdown. To this end, inflammation resembling that encountered by dentists is induced in animals by allowing plaque to accumulate or placing cotton ligatures on the gum. It is difficult to create standardized defects, but valuable observations can be made on the timings of extraction and implantation, healing periods and plaque control. The efficacy of treatments for preventing tissue loss can be assessed, as well as options for antimicrobial decontamination. Investigators can rely on the clinical relevance of the endpoints described here, which involve assessment by probing of peri-implant pockets, determining the level of the alveolar bone crest, the amount of bone–implant contact and the extent of inflammatory cell infiltrates. This remains a rich area for research in a growing area of practice. -

Compromised Bone Healing
The bone healing process in people can be affected by diseases such as Crohn’s disease, diabetes and colitis. It can also be affected by drugs such as bisphosphonates, which are used to treat weak bones in people with osteoporosis, Paget's disease and certain cancers. The role of animal models is enormous for investigating these disease conditions as well as other “risk factors” for compromised healing, such as radiotherapy, which is being increasingly used as early treatment of cancer in the community rises. Diabetes and intestinal disorders can be induced in rats, often using chemicals with toxic effects, but the diseases can take some time to develop. This chapter gives guidance on various timeframes for evaluating inflammatory processes, osseointegration and collagen cross-linking, and gives details on mechanical strength testing of dental implants and other candidate therapies. Different aspects of the compromised bone healing response can be observed using both the long bones (tibia and femur) and the maxilla of rats. This chapter also describes an adaptable study protocol for new investigators, with suitable endpoints such as bone to tissue ratio and bone to implant contact, to contribute to the body of knowledge on patients with compromised bone healing who are increasingly encountered in dental practice. -


Regulatory And Good Clinical Practice Aspects In Clinical Practice
based on the book chapter by Gudrun Denke Summary It is critical for researchers conducting patient-oriented research to attend to the regulatory aspects of their studies and employ good clinical practice (GCP) throughout. This chapter provides an overview of GCP in both Europe and the USA, with valuable summaries of the many processes involved when preparing to conduct trials on medical devices, drugs and biologics in regenerative surgery, with a particular focus on the content and scope of ISO 14155:2011. The regulatory documentation is described, such as investigator brochures, case report forms, and clinical investigation plans. They authors go into detail about meeting ethical, quality and national standards through appropriate clinical investigation planning and monitoring. They draw attention to the roles and functions of sponsors and principal investigators, as well as CE marking and trial termination. The importance of assigning risk to medical devices and products is also discussed, whereby absorbable materials, bone morphogenic proteins, dental lasers and subperiosteal implants are deemed to carry significant risks, while caries-removal solutions, fillers and traditional cushions pads are considered insignificant. The four phases of trials for drugs and biologics are also outlined, together with the need for a separate classification scheme for medical devices that comprises exploratory (first-in-human), confirmatory (safety and efficacy) and post-marketing follow-up (improvement) phases. Open full-text PDF (1.1 MB) -


Ethical Aspects In Clinical Research
based on the book chapter by Klaus Peter Rippe Summary This chapter deals with the moral and ethical minefield faced by all investigators conducting clinical experiments in patients. Obtaining histological material for studies of tissue regeneration can be ethically compromising, and any intervention may lead to burdens as well as benefits for patients, even after robust studies in animal models. In the absence of a clear concensus on morality or a simple philosophical stance, the authors focus on the principals of proportionality and equipoise, the potential for risk, international justice, and protecting patients from exploitation due to geographical or socioeconomic factors. They stress the importance of obtaining voluntarily consent from every participant, or surrogate decisions for vulnerable patients, and highlight the need for leaving no doubt about what might happen during the trial and the potential for harm. The obligation to help all patients poses unique issues: some are simply fixed, for example by comparing a new therapy with the current best one (rather than no therapy or placebo); and others are complex. However, it is acknowledged that when a particular trial arm offers more potential than one or more other arms of the study, any patient who gains a benefit also provides a means to benefit others. The doctorpatient relationship is also examined in this chapter, particularly clinicians who must recruit their own trial participants, for example when conducting preventive oral health trials among school-children. Open full-text PDF (1 MB) -


Development Of A Clinical Research Protocol
based on the book chapter by Maurizio S. Tonetti Summary Clinical investigations that fail to provide clear and relevant answers are a waste of valuable resources. This chapter describes how to avoid common pitfalls at the proposal stage, such as rejection by an ethics committee, or producing meaningless study results. With reference to ISO-14155-2011 throughout, the authors offer guidance on writing robust clinical protocols, formulating clinically relevant questions, and designing experiments that align directly with the clinical question. In the context of equipoise and ethical soundness, the practical aspects of patient selection, bias, sample size, trial arms and control groups, are addressed, as well as blinding and randomization, data collection and analysis, and obtaining meaningful outcome measures. Among these are patient-related outcomes (PROMs), which are increasingly important for identifying therapies that produce relatively less discomfort and better esthetics. When it comes to protocol design, prevention rather than cure is clearly preferable; among the issues raised are identifying potential errors and taking corrective action at the protocol stage; collaborating with all stakeholders including statisticians and administrators during the development stage; using the peer review process to improve chances of ethical approval; and allowing public and academic access to the protocol via trial registries, in order to aid recruitment, enhance collaboration, identify gaps in the research, and prevent duplication of studies. Open full-text PDF (1.1 MB) -


Management Of A Clinical Study
based on the book chapter by Jeanie Suvan Summary The focus of this chapter is on the appointment of a study manager to maximize the success of a clinical investigation. It explains the role of study managers (as distinct from principal investigators) in clinical trials, from initiation to study closure, applying project management principles from chairside to site facility. They use tailored planning strategies and tools including checklists, agendas, schedules, databases and spreadsheets, to deal with administrative and financial matters, standard operating procedures, timelines, logistics and auditing. Their involvement begins with translation of the protocol into reality and meeting regulatory requirements, through to data collection, adverse event recording and collation of documents in site files. The authors point out how good study managers identify limiting factors and barriers, prevent serious errors from occurring, and ensure all phases of the study flow smoothly. Their invaluable contributions include budgeting and tracking systems, devising formulae for calculating resources and clinic time, and contingency plans for coping with equipment failures and sickness absence. They contribute to team training, motivation and communication, and collaborate externally with ethics committee, funders, sponsors and report writers. They also help with patient recruitment, gaining informed consent, monitoring and retaining patients, and ensuring compliance. This information provides a compelling case for engaging a study manager. Open full-text PDF (1.1 MB) -

Authorship And Publication Of Research Findings
based on the book chapter by William V. Giannobile Summary This chapter describes how the volume of published research papers has risen over the last fifty years. The reasons it gives include not only dissemination of the findings and adding to the body of evidence but also the fulfilment of professional requirements -- for sponsors, for promotion and tenure. The author points to the valuable resources provided by the ICMJE (International Committee of Medical Journal Editors) and COPE (Committee on Publication Ethics), and lists what must be done when preparing a manuscript for submission. The tendency to include more authors is seen in all areas of research, including orodental, particularly with respect to dental practice-based research networks. Consequently, it is very important to be consistent and fair when assigning authorship, as distinct from contributorship. Here, the two roles are clearly distinguished, with a list of four ICJME criteria that must be met by authors. Reference is made to the publishing standards for common types of studies, such as CONSORT for randomized controlled clinical trials, which addresses how trials are designed, analyzed and interpreted; and STROBE for non-randomized clinical investigations, such as case reports, case series and cohort studies. The chapter also addresses issues such as over-citation, intellectual rights, gift authorships, redundancy, plagiarism, scientific misconduct and transparency, and points out the requirement by most peer-reviewed journals for international trial registration. Open full-text PDF (0.9 MB) -
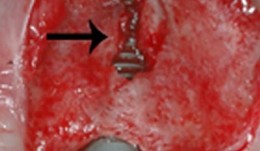

Study Protocols: Ridge Preservation
based on the book chapter by Suzanne Mason, Feng Wang, andDarnell Kaigler Summary This chapter explains the mechanisms of bone resorption and describes how to restore ridge deficiencies after tooth extraction and ensure a stable alveolar ridge for optimal implant survival, with a review of techniques and maintenance. The protocol was derived from a published study on the bone regeneration and stability after extraction of pre-molar or single-rooted anterior teeth. It defines the research question relating to a randomized controlled feasibility study, and cites how many patients are needed to undergo surgery. There are two treatment arms: one with autologous grafts using tissue repair cells (TRC) such as macrophages and mononuclear cells, derived from the patients bone marrow aspirates; others receive guided bone regeneration. The design, illustrated by a CONSORT diagram and clinical images of the procedures, incorporates phases for grafting/bone healing, implant osseointegration and biomechanical loading/restoration, with a 6-month follow-up consisting of postoperative care, specified evaluations and adverse-event recording. Primary and secondary endpoints are clearly defined, with assessments by CT, radiography, histology, the Misch bone density scale and torque testing. The recommended analysis is by KaplanMeier survival curves. The authors address the limitations of stem cell techniques, their isolation and expansion, and of transplantation of cells and grafts. Because biologicals are used, the authors recommend forming a data safety monitoring board (DSMB). Open full-text PDF (1.4 MB) -
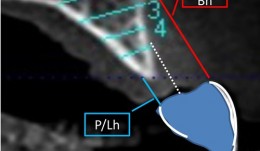

Study Protocols: Horizontal Ridge Augmentation
based on the book chapter by Nikos Donos and Nikos Mardas Summary This protocol focuses on measuring radiographic bone levels on the buccal side of augmented areas around implants after horizontal ridge augmentation, rather than at interproximal sites (a limitation of most published studies). The design offers a standardized means to assess graft materials, bone fillers and bone substitutes for horizontal ridge preservation, allowing comparisons of resorbable and non-resorbable materials and natural and synthetic membranes. It includes two randomized controlled trials for patients with bone defects from destructive gum disease who need local ridge augmentation before implantation. One trial uses a simultaneous technique whereby implants are placed in bone augmented with a new bone substitute plus a new or standard membrane, or a standard collagen membrane plus deproteinized bovine bone mineral; this trial comprises two phases surgical implantation and observation. The other trial is a staged technique comprising a new block-bone graft/substitute plus a new or standard membrane, and autogenous block bone graft plus a standard collagen membrane; this has a third phase. A 1-year timeline and 5-year follow-up is suggested for both trials. The authors cite various histologic and radiographic endpoints, photon bubble oscillations, peri-implant bone preservation, implant survival, patient morbidity, adverse events and patient-related outcomes. Images are provided of the change in bucco-palatal/lingual alveolar ridge width 6 months after the procedure. Open full-text PDF (1.3 MB) -
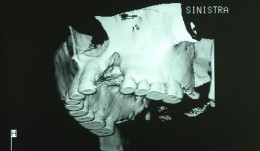

Study Protocols: Vertical Ridge Augmentation
based on the book chapter by Isabella Rocchietta, David Nisandand Massimo Simion Summary This chapter reviews the need for standardized research into vertical ridge augmentation procedures because restoring bone volume after trauma or disease is clinically challenging; the outcomes are unpredictable, the complication rates are high and success is limited. The presented protocol involves initial augmentation surgery comprising a composite graft of autogenous bone and deproteinized bovine bone, with tenting screws and a PTFE membrane overlay, followed 6 months later by surgical removal of the membrane and screws and placement of an implant. Guided bone regeneration is compared with a gold standard two-stage procedure using autogenous bone strips with deproteinized bovine particles and a titanium-reinforced membrane. The timeline incorporates a 6-month evaluation phase and further evaluation 4 months later, with a 5-year follow-up. Patient numbers and selection criteria are defined, along with suitable endpoints, care routines and the use of cone-beam CT to assess bone gain and quality. The descriptions are illustrated by CT images, radiographs and clinical photographs that show preparation of the donor and implant sites in both surgical phases. The evaluation phase relates to responses of the defect over time in terms of resorption of graft particles, soft tissue swelling, inflammation, and bone destruction and formation. What the protocol aims to do is improve outcomes for patients with traumatic tooth extractions, jaw damage, endodontic infections and failed implants. Open full-text PDF (1.4 MB)