-


Goold Laboratory Practice (GLP)
All preclinical studies on therapies, procedures and materials intended for use in people must be carried out in accordance with the principles of good laboratory practice (GLP). This chapter gives an overview on the history and scope of GLP, its requirements for the submission of information, and its role in producing safe, high-quality products. Adhering to these principles can be challenging during this era of globalization, especially because different countries have their own regulatory authorities, inspection intervals, legal frameworks and administrative practices. Differences occur not only at a country level, but also at organizational, facility, staff and laboratory levels, resulting in enormous variability in the planning, monitoring, auditing, reporting and archiving of studies. This chapter outlines the responsibilities of all key players in the certification process – study directors, sponsors, principle investigators, archivists, and technical and scientific staff – and suggests improvements that support the flow, sharing and integration of study information on a global scale. A standardized approach at international, national and organizational levels will minimize barriers to information exchange and trade, and facilitate distribution of reproducible, traceable data, with a continuous line of evidence on safety and efficacy of individual technologies. -


Ethical Considerations for Performing Research in Animals
Laboratory tests have limited value for assessing the safety and effectiveness of new therapies in people. Tests must also be conducted on animals that resemble humans, both biologically and developmentally. We generally acknowledge that certain animals may be caught and sold, kept in captivity, or eaten, but using animals to meet human needs is as an area of huge controversy. This chapter gives a broad perspective on the ethical basis for animal experiments, drawing from the modern “common sense” view and several longstanding philosophical theories. Moral status is considered, alongside integrity, autonomy and dignity of animals, and their ability to reason, to form memories, and to experience pleasure or pain. Smaller animals, such as mice, rats and rabbits are essential for proving a basic principle or concept. Larger animals, such as goats, pigs, sheep, dogs and monkeys, are used more sparingly, not least because of the costs involved in their care; they are necessary because of their greater similarity to humans and thus are more relevant to advancing clinical practice. However, conflicts of interest tend to be larger with animals that are more similar to humans. Primate and dogs tend to evoke our compassion more strongly than rodents or animals we commonly eat. The authors provoke thought on this subject through examples in which the interests of humans are weighed up with those of animals in studies of cosmetics, childhood leukemia and dental defects. -
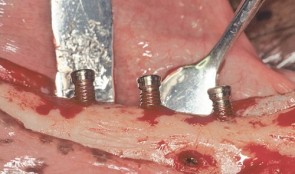

Vertical Ridge Augmentation
Vertical augmentation is essential for restoring bone defects in patients with destructive gum disease, before rigid fixation of implants or grafts. However, in practice it is associated with high complication rates and limited success. Thus it is important to refine the techniques used by dental surgeons through preclinical research. This chapter looks at various animal studies, and focuses on one specific validated, reproducible and reliable protocol. This involves creating a saddle-like, bony, critical-size defect in the mandible of dogs, in which sufficient time is allowed for the defect to become chronic, thus mimicking bone atrophy in humans. Vertical bone gain is assessed following placement of a tissue-engineered block. Surgical and flap management techniques can be tested, as well as different biologics, devices, scaffolds, membranes, implants and screws. Assessment relates to the initial defect, as well as responses over time relating to resorption of graft particles, soft tissue swelling and inflammation and bone destruction and formation. Osseointegration is quantified to indicate success or failure of the intervention. The protocol can also be used to compare analytical methods. The methods have fairly predictable outcomes, and are applicable to patients with traumatic tooth extraction, jaw damage, endodontic infections and failed implants. -
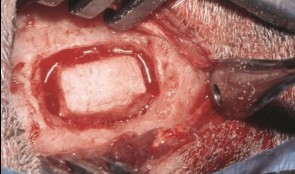

Sinus Floor Augmentation
The maxillary sinuses are located inside the cheekbones, above the upper jaw, from the second premolar area to the wisdom teeth. In some people with tooth loss, the sinuses are too close to the upper jaw for dental implants to be placed; in others, bone may have been reabsorbed because of gum disease. In either case, bone height can be restored using a sinus lift – an increasingly common technique in dental practice. A small hole is made in the bone beneath the gum and the membrane lining the sinus is pushed away from the jaw to create a space into which bone graft material can be packed. Implants can then be placed after the graft has integrated with the natural tissue. Preclinical models are necessary for testing bone grafts from different sources, and different bone substitution materials, biologically active coatings, growth factors, and implant types. The vascularization process can also be investigated. This chapter discusses the merits of various animals for studying the pathology and repair of sinus defects, with a preference for those with similar sinus structures, bone loading characteristics and remodeling processes to humans. Osseointegration of graft materials can take many months, so the protocols here provide a framework of suitable timings and sequences of surgical procedures, including the placing of implants at same time as augmentation. Suitable endpoints are defined too to assist investigators in planning and reporting on reproducible, relevant outcomes. -
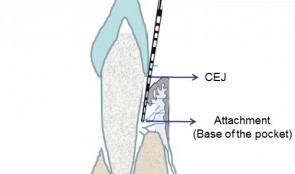

Examiner Standardization And Calibration
based on the book chapter by Hatice Hasturk and Mary Ann Cugini Summary The emphasis of this chapter is on examiner--therapist alignment and standardization when conducting controlled clinical trials, especially those with multicenter settings. It begins with definitions of various key research team members and follows by giving specific advice on assessing regeneration by periodontal and bone probing, with reference to parameters relating to probing depth, the cemento--enamel junction, gingival margins, keratinized gingiva, tooth mobility, clinical attachment and alveolar bone gain. The authors explain the relevance of bleeding, plaque and gingival health indices, and the stress the importance of selecting suitable reference points and scoring scales. Detail is provided on the techniques of mucogingival regeneration, alveolar ridge augmentation and implant insertion, and factors such as wound healing. There are protocols for alignment and agreement sessions, and recommendations for conducting regular realignment sessions, with tailored training and calibration, in order to ensure consistency in skills and performance of the team, in data capture and interpretation, and in use of instruments and scoring systems. Practical tips are given on various aspects of the study including set up and timings, examiner--observer agreement and inter-class correlation, with guidance on organizing the research team, assessment plans and statistical analysis. The overall aim of the chapter is to ensure production of high-quality data with minimal uncertainty. Open full-text PDF (1.4 MB) -


Patient-Reported Outcome Measures
based on the book chapter by Colman McGrath Summary There has been a surge of interest in patient-reported outcome measures (PROMs) in all areas of clinical research. Assessing the patients own perceptions of their health, quality of life, functional ability and experience of pain provides very valuable information on the success of an intervention. This chapter describes the development of some commonly used instruments, and summarizes their limitations and suitability for different studies in implant surgery and tissue regeneration. It advises investigators on the selection of different types generic, condition-specific, dimension-specific and utility measures. The authors explain why general (global) instruments like the popular Short Form SF-36 questionnaire have limited sensitivity for oral outcomes, suggesting several condition-specific tools that yield far more specific data and are quick to complete, making them suitable for studies in busy clinic settings and large numbers of patients. The authors describe the role of health utility indexes, which allow patients to rank the importance of items affecting their quality of life, or permit costbenefit analysis of an intervention. The instruments are discussed in the context of their appropriateness for a particular study, their acceptability to patients, validity, reliability and reproducibility, and their responsiveness to change. In terms of interpretation, emphasis is placed on the challenge of identifying the minimally clinically important difference (MCID). Open full-text PDF (0.9 MB) -
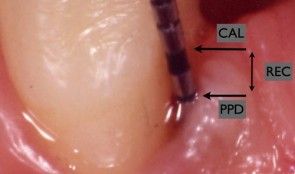

Endpoints In Oral And Maxillofacial Regeneration Clinical Trials
based on the book chapter by Mariano Sanz and Fabio Vignoletti Summary Selection of endpoints, or outcome measures, can be daunting for researchers because of the huge variation in disease processes and outcomes relating to implants, surgery and tissue regeneration, and factors such as the research question and study design. This chapter advises investigators on the selection of valid, patient-applicable, sensitive, specific, ethical endpoints, that do not damage tissue integrity. It defines the terms, and distinguishes between true and surrogate and primary and secondary endpoints. It explains about different types biological, clinical, psychological and economic -- and summarizes their limitations and value, such as biological measures for disease etiology and progress, and clinical measures for assessing survival and function. The authors focus on common parameters relating to periodontal probing, bone radiography and patient-related outcomes, and describe the relevance of numerous specific endpoints in studies of localized gingival recession, soft tissue augmentation and mandibular furcation regeneration. They also review endpoint selection in implant therapy, for evaluating bone regenerative therapies, protocols for socket preservation and immediate implantation, lateral and vertical bone augmentation, and implant-supported restorations. Throughout the chapter, the chosen endpoints are related to the wider aspects of patient selection, randomization and compliance, data collection and observer variation, error proneness and reporting of results. Open full-text PDF (2.3 MB) -

Authorship And Publication Of Research Findings
based on the book chapter by William V. Giannobile Summary This chapter describes how the volume of published research papers has risen over the last fifty years. The reasons it gives include not only dissemination of the findings and adding to the body of evidence but also the fulfilment of professional requirements -- for sponsors, for promotion and tenure. The author points to the valuable resources provided by the ICMJE (International Committee of Medical Journal Editors) and COPE (Committee on Publication Ethics), and lists what must be done when preparing a manuscript for submission. The tendency to include more authors is seen in all areas of research, including orodental, particularly with respect to dental practice-based research networks. Consequently, it is very important to be consistent and fair when assigning authorship, as distinct from contributorship. Here, the two roles are clearly distinguished, with a list of four ICJME criteria that must be met by authors. Reference is made to the publishing standards for common types of studies, such as CONSORT for randomized controlled clinical trials, which addresses how trials are designed, analyzed and interpreted; and STROBE for non-randomized clinical investigations, such as case reports, case series and cohort studies. The chapter also addresses issues such as over-citation, intellectual rights, gift authorships, redundancy, plagiarism, scientific misconduct and transparency, and points out the requirement by most peer-reviewed journals for international trial registration. Open full-text PDF (0.9 MB) -


Management Of A Clinical Study
based on the book chapter by Jeanie Suvan Summary The focus of this chapter is on the appointment of a study manager to maximize the success of a clinical investigation. It explains the role of study managers (as distinct from principal investigators) in clinical trials, from initiation to study closure, applying project management principles from chairside to site facility. They use tailored planning strategies and tools including checklists, agendas, schedules, databases and spreadsheets, to deal with administrative and financial matters, standard operating procedures, timelines, logistics and auditing. Their involvement begins with translation of the protocol into reality and meeting regulatory requirements, through to data collection, adverse event recording and collation of documents in site files. The authors point out how good study managers identify limiting factors and barriers, prevent serious errors from occurring, and ensure all phases of the study flow smoothly. Their invaluable contributions include budgeting and tracking systems, devising formulae for calculating resources and clinic time, and contingency plans for coping with equipment failures and sickness absence. They contribute to team training, motivation and communication, and collaborate externally with ethics committee, funders, sponsors and report writers. They also help with patient recruitment, gaining informed consent, monitoring and retaining patients, and ensuring compliance. This information provides a compelling case for engaging a study manager. Open full-text PDF (1.1 MB) -


Development Of A Clinical Research Protocol
based on the book chapter by Maurizio S. Tonetti Summary Clinical investigations that fail to provide clear and relevant answers are a waste of valuable resources. This chapter describes how to avoid common pitfalls at the proposal stage, such as rejection by an ethics committee, or producing meaningless study results. With reference to ISO-14155-2011 throughout, the authors offer guidance on writing robust clinical protocols, formulating clinically relevant questions, and designing experiments that align directly with the clinical question. In the context of equipoise and ethical soundness, the practical aspects of patient selection, bias, sample size, trial arms and control groups, are addressed, as well as blinding and randomization, data collection and analysis, and obtaining meaningful outcome measures. Among these are patient-related outcomes (PROMs), which are increasingly important for identifying therapies that produce relatively less discomfort and better esthetics. When it comes to protocol design, prevention rather than cure is clearly preferable; among the issues raised are identifying potential errors and taking corrective action at the protocol stage; collaborating with all stakeholders including statisticians and administrators during the development stage; using the peer review process to improve chances of ethical approval; and allowing public and academic access to the protocol via trial registries, in order to aid recruitment, enhance collaboration, identify gaps in the research, and prevent duplication of studies. Open full-text PDF (1.1 MB)