-


THE CASE BOX
Osteology Foundation -
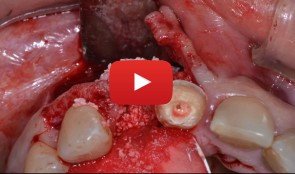

Treatment of a fenestration defect with GBR and CT graft
João Batista Cesar Neto -

-

-


BIO-ACTIVATION OF DEPROTEINISED BOVINE BONE MINERAL (DBBM) AND NON-CROSS-LINK COLLAGEN MEMBRANES BY THE USE OF GROWTH FACTORS EXTRACTED FROM FRESH AUTOGENOUS BONE CHIPS
Objectives: Autogenous bone grafts are the gold standard for bone augmentation procedures with the ability to release growth factors. These growth factors can be isolated into a "bone-conditioned medium" (BCM). No effort has been made to utilise the growth factors from fresh bone chips in combination with biomaterials to improve bone regeneration. This study aimed to investigate the ability of collagen barrier membranes and DBBM treated with BCM to affect cell behaviour. Methods: Cortical bone chips were harvested from fresh pig mandibles with a bone scraper and placed into plastic dishes containing serum-free culture medium (5g of bone chips per 10mL of medium) for 24 hours. Natural collagen membranes (Bio-GideTM/®) were incubated with BCM for various times. Membranes were also (i) incubated for 4 hours with recombinant TGF-β1; (ii) exposed to ultraviolet light prior to BCM incubation; (iii) pre-wetted for 15 minutes with phosphate buffer saline (PBS) prior to BCM incubation; or (iv) dried and stored at room temperature for 7 days after BCM incubation. After incubation, the membranes were vigorously washed with PBS. DBBM particles (Bio-OssTM/®) were coated with BCM for 5 minutes prior to cell seeding. Gingival fibroblasts or bone-marrow-derived stromal cells (ST2 cells) were seeded on the collagen membranes and DBBM particles, respectively. Messenger RNA levels of BCM target genes were analysed by qRT-PCR using adrenomedullin (ADM), pentraxin 3 (PTX-3), interleukin 11 (IL-11) and proteoglycan-4 (PRG-4). The morphology and viability of cells seeded onto collagen membranes was evaluated. Further, DBBM with and without a BCM coating was compared in terms of cell recruitment, adhesion, proliferation and qRT-PCR for osteoblast differentiation markers (including Runx2, COL1A2, ALP and OCNAlizarin red stain was used to assess mineralisation. The student‘s t-test was used for analysis. Results: Incubation of collagen membranes with BCM for at least 1 minute reduced fibroblast ADM and PTX-3 expression, and increased IL-11 and PRG-4 expression. The four different membrane treatments (i–iv) also provoked significant changes in gene expression. Likewise, conditioned medium from demineralised bone chips caused similar changes in gene expression compared to BCM. BCM did not alter the viability or morphology of gingival fibroblasts on collagen membranes. Coating BCM on DBBM particles improved cell migration of ST2 cells and led toa two-fold increase in cell adhesion. No significant increase in cell proliferation was observed, but BCM significantly increased mRNA levels of COL1a2, ALP and OCN at 3 days post-seeding. A three-fold increase in alizarin red staining was observed on DBBM particles that were pre-coated with BCM. Conclusions: Collagen membranes rapidly adsorb the TGF-β activity of bone chips, and pre-coating DBBM with BCM enhances the osteoconductive properties of DBBM by mediating osteoblast recruitment, attachment and differentiation towards an osteoblast phenotype. These cellular effects of BCM, in combination with biomaterials, might contribute to the overall process of guided bone regeneration. Further animal studies are needed to characterise the added benefit of BCM as an autogenous growth factor for combination therapies. -


A COMPARATIVE ANALYSIS OF DEMINERALISED FREEZE-DRIED BONE (DFDBA), FRESH FROZEN BONE ALLOGRAFT (FFBA) AND AUTOGENOUS BONE GRAFT (AU)—A HISTOLOGIC STUDY IN RABBITS
Objectives: There are different clinical applications for bone grafts in alveolar reconstructions and difficulties in achieving vertical osseous increase. This study was to make a comparative histological evaluation of DFDBA, FFBA, AU and blood clot (CO) on vertical guided bone regeneration (GBR) in rabbit calvaria. Methods: Nine rabbits were used. One was the primary bone graft donor and eight were GBR models, whereby 32 titanium cylinders were fixed to the calvaria and randomly filled with DFDBA, FFBA, AU or CO. The animals were killed 13 weeks later and the contents of the cylinders were analysed histomorphologically and histomorphometrically to quantify the total area (AT) of newly formed tissue, new bone (NB) and residual graft (RG) particles. Results: Mean values of AT were significantly higher for DFDBA and FFBA in the order DFDBA = FFBA > AU > CO. New bone formation with DFDBA and FFBA was better than with AU or CO. There were more RG particles in the DFDBA models, in the order FFBA > DFDBA = AU = CO (p values Conclusions: Allografts containing DFDBA and FFBA can be considered beneficial for achieving new vertical bone formation. -


Esthetic and Restorative Dentistry - Esthetic Post Systems
Terry, Douglas A. -
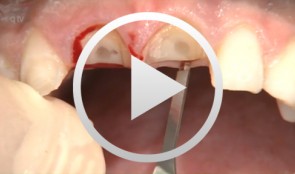

Socket-shield surgery on two central incisors
Hürzeler, Markus B. -

Esthetic and Restorative Dentistry - Ceramic Materials
Terry, Douglas A. -
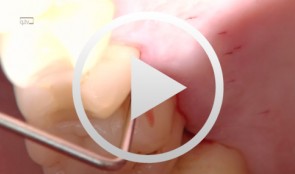

Treatment of a palatal class II furcation
Marggraf, ErwinOutline: - Reflecting a flap - Cleaning. - Fraction 3 - Fraction 2 - Fraction 1 - Wound closure List of materials All materials required for producing PRGF (BTI Germany) Bone replacement materials Geistlich Biomaterials Surgical instruments, Aesculap Suture materials, Ethicon