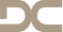-


WHEN THINGS GO WRONG AFTER SINUS FLOOR ELEVATION
Objectives: Lateral sinus floor elevation (LSFE) is a procedure that allows insertion of dental implants in severely atrophic alveolar ridges in posterior maxilla. Early failure is possible during the osseointegration period and late failure is possible during loading, due to chronic inflammation. Additional LSFE is sometimes needed to restore normal chewing function. We present a case series in which additional LSFE was successfully performed after the occurrence of early and late complications. Methods: In four patients treated in the Department of Maxillofacial and Oral Surgery at the University Medical Centre Ljubljana, four implants were inserted in the posterior maxilla with LSFE using deproteinised bovine bone mineral (DBBM; Bio-OssTM/® and collagen membrane Bio-guideTM/®). Three implants were simultaneously inserted during LSFE. In one patient, implants were inserted 4 months after LSFE. Three implants in three patients were removed during re-entry and placement of healing abutments. The fourth implant was fractured after 5 years of function due to undiagnosed and untreated peri-implantitis. Lost implants in three patients were removed, the implant sites were debrided, and the wounds left to heal for 4 months. Then the fractured implant was partially removed. The apical part was osseointegrated and left in place. In two patients, oro-anthral fistula developed, which was closed by advancement of buccal flap. After healing for 4 months, cone-beam CT was performed and additional LSFE was done in all four patients with simultaneous implant placement with deproteinised bovine bone mineral (DBBM) and collagen membrane. In three patients, fibrous encapsulation of DBBM was found and removed and purulent discharge occurred in two patients. Antibiotics, decongestants and analgesic therapy were given to all patients in the postoperative period. Results: Mean alveolar ridge height before additional LSFE was 3.8mm (range 4.0–3.5mm). In all patients, the Schneiderian membrane was intact during the second elevation. All implants osseointegrated uneventfully. Healing abutments were inserted after 4 months of healing and prosthetic constructions were delivered according to a standard prosthetic protocol. Conclusions: Despite the fact that LSFE is predictable, failures can occur. With a proper surgical protocol in place, additional LSFE can be performed once again. During the second LSFE, all infected DBBM particles have to be removed and the integrity of Schneiderian membrane must be maintained. -


THE EFFECT OF BIOENGINEERED MUSSEL GLUE AS A bone morphogenetic protein (BMP)-2 CARRIER FOR BONE REGENERATION
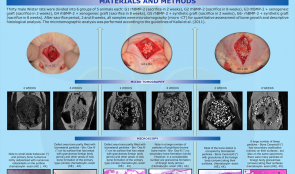
Objectives: Recombinant human (rh)BMP-2 is known to induce bone formation by stimulating osteoblast differentiation. Mussel adhesive proteins (MAPs) secreted from mussels have been suggested as suitable adhesives for tissue engineering and medicine. In this study, rMAP was used to immobilise rhBMP-2 on a titanium mesh (Ti-mesh) surface, and the osteoinductivity of the BMP-2/rMAP on Ti-mesh surfaces was investigated in vitro and in vivo. Methods: (i) Expression and purification of rMAP: Hybrid rMAP was expressed in Escherichia coli and the proteins were harvested for purification after centrifugation. The rMAP was extracted using 25% (vol/vol) acetic acid, and endotoxin was removed by sequential purification. The purity of rMAP was assessed by 12% (wt/vol) sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). (ii) BMP-2 immobilisation using rMAP on Ti-mesh surfaces: For in vitro analysis, surfaces were coated with 50µg/cm2 of rMAP solution after removing all organic molecules from the Ti-mesh surface. To immobilise BMP-2 on Ti-mesh surfaces, rMAP solution was mixed with 50µg/mL of rhBMP-2 in sodium bicarbonate (pH 0.8), and coated on the mesh surfaces as described previously. (iii) Animal surgery for in vivo bone regeneration ability assays: Each kind of Ti-mesh surface was tested on male Sprague-Dawley rats (n = 6) weighing 250–300g. The rat calvaria were exposed through skin and periosteum incisions using a #15 surgical blade, and circular bone defects were punched using a 8-mm diameter trephine drill under saline irrigation. Two short screws were used to fix each Ti-mesh (prepared just before surgery) over the defect. (iv) Radiographic and histological assessment was made of in vivo bone regeneration. (v) In vitro cell attachment and proliferation assays were performed. (vi) In vitro osteoblast differentiation assays included phenotypic differentiation assays and osteogenic gene expression analysis. Results: Through micro-CT analysis and histological evaluation, the BMP-2/rMAP-coated titanium surfaces displayed improved new bone formation in the rat calvarial defect model compared to rMAP-coated and uncoated surfaces. In addition, cellular behaviours (e.g. adhesion and proliferation) of mouse pre-osteoblast MC3T3-E1 cells were significantly increased on Ti-mesh surfaces coated with BMP-2/rMAP and rMAP. The BMP-2/rMAP-coated surface showed the highest degree of osteogenic differentiation. Messenger RNA expression of osteogenic differentiation marker genes was upregulated in MC3T-E1 cells on BMP-2/rMAP-coated Ti-mesh surfaces. Conclusions: Recombinant MAP is a promising biomaterial for BMP-2 immobilisation on titanium surfaces and guided bone regeneration with enhanced osteoinductivity -


PERI-IMPLANT ALTERATIONS AFTER IMMEDIATE IMPLANT PLACEMENT AND PROVISIONALISATION IN SOCKETS PRESENTING WITH A FACIAL BONE DEFECT WITH SOFT TISSUE GRAFTS—A PROOF-OF-PRINCIPLE STUDY
Objectives: The study aimed to evaluate soft and hard tissue alterations following flapless immediate implant placement and provisionalisation in sockets with a facial bone dehiscence that were reconstructed and received different soft tissue grafts in a proof-of-principle, randomised clinical trial of 1-year duration. The hypothesis was that the use of a soft tissue graft would reduce gingival margin apical migration at the facial aspect. Methods: Twenty-four patients with a single failing maxillary incisor presenting with a facial bone dehiscence were selected. After tooth extraction and narrow implant installation with a torque in excess of 32 N/cm, patients were randomised into three groups (n = 8): a control group (CTL) with no soft tissue graft; a collagen matrix (CM) group using Mucograft[tm]; and a connective tissue graft group (CTG) using subepithelial connective tissue from the palate. Sockets were reconstructed with inorganic bovine bone mineral containing 10% of porcine collagen (Bio-Oss Collagen[tm]) and a non-cross-linked collagen membrane (Bio-Gide[tm]). Additionally, a provisional with a concave subgingival contour an no occlusal contacts was installed in the implants. Clinical, photographic, aesthetic and tomographic analyses were performed by a blinded examiner at baseline, 6 months and 12 months to evaluate tissue alterations. Results: All implants were successfully osseointegrated and a few minor complications occurred. Gingival margin apical migrations of 0.72 ± 0.57mm, 0.42 ± 0.60mm and –0.04 ± 0.3mm occurred respectively in groups CTL, CM and CTG; this was significantly lower in the CTG group than the CTL group. Soft tissue thickness was significantly increased at 12 months for CTL (2.11 ± 0.60mm), CM (2.1 ± 0.54mm) and CTG (3.04 ± 0.61mm. The CTG group demonstrated significantly greater thickness and alveolar ridge contour in comparison to CTL and CM. No significant differences were observed between groups in terms of aesthetic evaluation, papilla apical migration, and height and thickness of the bone in contact with the facial aspect of the implant. In five patients, it was not possible to completely reconstruct the facial bone wall; this was related to the transverse format of the ridge defect, influencing both facial bone height and thickness. Conclusion: Minimal soft tissue alterations occur after immediate implant and provisional placement in sockets with buccal bone dehiscences 1 year after the surgical procedure. The use of connective tissue grafts avoids gingival margin apical migration, and provides better contour of the alveolar ridge and greater thickness of the soft tissue at the buccal aspect of the implant. -

MICROTOMOGRAPHY AND MICROSCOPIC ANALYSIS OF THE BEHAVIOR OF RECOMBINANT BONE MORPHOGENETIC PROTEIN-2 (rhBMP-2) ASSOCIATED WITH BIOMATERIALS IN REPAIRING DEFECTS CRITICS ON RAT CALVARIUM
Objectives: Tissue engineering is a rapidly growing field concerned with providing biological replacement therapies for damaged tissues and organs. This study compared bone formation induced by rhBMP-2 associated with two particulate bone substitutes in critical-size defects in rat calvaria. Methods: Thirty male Wistar rats were divided into six groups of five animals: Group 1 rhBMP-2 (sacrificed after 2 weeks); Group 2 rhBMP-2 (sacrificed after 8 weeks); Group 3 rhBMP-2 + xenogeneic graft (sacrificed after 2 weeks); Group 4 rhBMP-2 + xenogeneic graft (sacrificed after 8 weeks); Group 5 rhBMP-2 + synthetic graft (sacrificed after 2 weeks); and Group 6 rhBMP-2 + synthetic graft (sacrificed after 8 weeks). All samples obtained underwent microtomography (micro-CT) for quantitative assessment of bone growth and descriptive histological analysis. Micro-CT analysis was performed according to the guidelines of Kallai et al. (2011). Results: In the interval between 2 to 8 weeks, group 2, group 4 and group 6 showed a significant increase in bone mineral density. After 2 weeks, group 3 and group 5 showed higher bone mineral density than group 1. After 8 weeks, the rh-BMP-2 alone (group 2) showed higher bone mineral density than groups associated with biomaterials (group 4 and group 6). The percentage of newly formed bone was significantly increased after 8 weeks in the groups in which rhBMP-2 was associated with biomaterials (group 4 and group 6) compared to group group 3 and group 5 after 2weeks. The two evaluation methods used in this study were effective. Conclusion: We conclude that xenogenic material and synthetic graft associated with rh-BMP-2 have the same effect on density and volume of newly formed bone. When used alone, rhBMP-2 can be applied with the same results, with bone growth that is similar to that seen in the groups with biomaterials, and statistically higher bone density than the groups with biomaterials. -


AUTOGENOUS GRAFTS IN BLOCK VERSUS XENOGENEIC GRAFT BLOCK—A CLINICAL STUDY IN HUMANS
Objectives: The aim of this study was to evaluate the stability (bone resorption) of autogenous and xenogeneic grafts in block and the primary stability of implants in the grafted areas. Methods: Eight patients were selected with two defects of bone thickness in the anterior maxilla. A graft of autogenous mandibular branch block was fixed on one side and xenogeneic (Bio-Oss Block[tm]) on the other; both were covered with a collagen membrane (Bio-Gide[tm]). Before and after graft fixation, vestibular–palatal bone thickness was measured on the mesial expanded head-set screw using a thickness gauge. After 6 months, another clinic measure of bone thickness was made in the same place, and a Cone Morse taper Alvim 3.5x10mm implant was placed (Neodent) in each graft. Primary stability was measured with a surgical wrench at the time of implant placement. For tomographic evaluation, tests were performed preoperatively, immediately postoperatively, and 6 months later. CT scans were made of the sagittal graft on the centre of the bone block and of the Bio-Oss Block[TM] and linear measurements were recorded. Results: The mean post-graft thickness at 6 months in autogenous grafts was 7.4mm, with an initial mean of 3.4mm and resorption at 2.6%; for the Bio-Oss Block[tm] the means were 8.9mm and 3.3mm, with resorption of 7.3%. Tomographic analysis at 6 months in autogenous grafts showed mean post-graft thickness of 7.8mm, with an initial mean of 3.7mm, and resorption of 0%. For the Bio-Oss Block[tm] the means were 9.3mm and 3.6mm and 2.1%, respectively. The mean implant insertion torque averaged 32±22N/cm in patients receiving autograft and 18±9N/cm in those receiving xenogeneic grafts. Conclusion: The use of xenogeneic bone graft block achieves a level of thickness and primary stability that indicates successful treatment. -


SOCKET PRESERVATION FOR IMPLANT-SITE MANAGEMENT USING PLATELET RICH FIBRIN (PRF)
Objectives: Traumatic loss of a natural tooth in the aesthetic zone demands immediate attention. One predictable option is socket seal with relined natural tooth, which helps to preserve the gingival parabolic contours and papilla (essential for maintaining natural simulation of soft tissue). Using natural tooth as a socket seal helps to maintain a dentogingival soft tissue collar and initiate a tissue healing process similar to that of tooth replantation. This report describes a patient with trauma-induced loss of an anterior tooth. Methods: The avulsed natural crown was sectioned and used as a socket seal, splinted with the patient’s adjacent teeth. After 3 months, an Ankylos[tm] implant was placed with immediate restoration over the final abutment using the same natural tooth as pontic. Results: Healing was uneventful, with a good emergence profile and healthy papilla at 3 months before placement of the implant. After follow-up of 6 months, vertical height of papilla and bone-crest-to-bone-contact distance were maintained. Conclusion: Replacement of missing natural tooth in aesthetic zone is always a clinical challenge. Simulating the exact dimensions of the lost tooth – especially on the cervical part of the new provisional restoration – is expected to optimise healing and promote a natural-looking emergence profile. The natural dental crown, connected to an implant instead of the root, is applied for tight sealing of the wound. When a tooth is not available because of a traumatic impact, a naturally dimensioned crown restoration is an alternative wound sealant. -


A STANDARD INDEPENDENT BONE REGENERATION MODEL FOR TISSUE ENGINEERING AND OSSEOINTEGRATION
Objectives: Functional tooth replacement and bone regeneration are a routine part of daily practice in modern dentistry, but there is no reproducible and relatively inexpensive animal model for multiple testing. We aimed to develop a new model for evaluating bone regeneration, osseointegration and critical size defects. Methods: We used a special guided approach at the level of the C4–C5 vertebrae in Wistar rats after cutting the tail for parallel placement of customised titanium implants. Bone remodelling around the implants was evaluated after 4–16 weeks. Biomechanical properties were characterised by measuring the maximum extraction force and using micro-CT, histomorphometry and resonance frequency analysis. Critical size defects for bone tissue engineering and implant placements for titanium body experiments were evoked by drilling each of the first four caudal vertebrae from the side, thus allowing multiple comparisons, and micro-CT and histology were used to evaluate bone growth. Results: The percentage regeneration was only of 20% ± 5% when bone grafting particles alone were used to fill defects. Bio-Oss[tm] was used for positive controls but the material was radio-opaque and could not be assessed by micro-CT. Histology revealed collagen network formation in defects filled with bone grafting material, but not in those with Bio-Oss[tm]. Immunohistochemical assays confirmed the presence of bone markers such as osteocalcin and type I collagen, and the presence of bone cells of both human and rat origin. A positive correlation was found between measurements made using various in vivo techniques(r=0.8498). Statistically significant increases were found during evaluations at 4, 8, 12 and 16 weeks in terms of strength of osseointegration. Histomorphometry and micro-CT showed new bone formation on the implant surfaces, suggesting the direct connection occurring between bone and titanium. Conclusion: These results support the use of the caudal vertebrae bone regeneration rat model as a useful standard for preclinical evaluation of tissue engineering and osseointegration. -


AN OLD-SCHOOL SOLUTION TO A NEW-AGE PROBLEM—RIDGE MAPPING FOR IMPLANT PLANNING
Objectives: Understanding the bony anatomy at the site of implant placement is essential for implant success. Computed tomography (CT) is generally considered the gold standard for assessing bone morphology, however there are situations in which it is not practical. The ridge-mapping technique has been advocated as a convenient way to assess site suitability for implant placement, and was used in this patient to plan a single dental implant. Methods: An unrestorable maxillary lateral incisor tooth was extracted and the socket was preserved using Bio-Oss[tm] and a Bio-Gide[tm] membrane. This healed undisturbed for 2 months, then alginate impressions were taken and plaster study models fabricated. A mapping stent was fabricated from the plaster model using rigid acrylic material to ensure stabilisation[stability?]. The stent overlapped the buccal and palatal mucosal tissue. Holes were drilled in the stent at 2mm, 4mm, 6mm and 8mm from the alveolar crest, allowing a size 20 endodontic file to pass through. At each pre-drilled site, the depth was measured from the acrylic stent surface to the bone. The cast was then sectioned and measurements were transferred to the cast to provide an outline of the underlying bone. Following clinical assessment and outline measures, a bone level Strauman implant of 3.3-mm diameter and 12-mm length (SLActive was planned. Local anaesthesia was administered before making a slightly palatal incision and reflecting a full-thickness mucoperiosteal flap. The alveolar ridge was measured using a caliper. The osteotomy was prepared using standard drills and the implant was placed 3mm from the cervical margin of the proposed restoration. Results: Ridge mapping and direct measurement with calipers yielded similar dimensions of the alveolar crest. Conclusion: This report demonstrated that ridge mapping using endodontic files and a model-based template gives an accurate representation of the underlying alveolar bone. This technique may be suitable when standard radiographic techniques are not possible. -
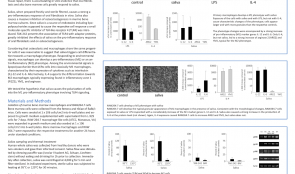

SALIVA SUPPORTS THE FORMATION OF PRO-INFLAMMATORY MACROPHAGES IN MURINE BONE MARROW CULTURES
Objectives: The process of oral wound healing requires a temporary inflammatory reaction. Saliva can support this process, as reported previously, by provoking a strong inflammatory response in oral fibroblasts, while suppressing osteoclastogenesis in bone marrow cultures. Bone marrow cells also give rise to a heterogeneous cell population of macrophages which are also a involved in wound healing. Lipopolysaccharide (LPS) and interleukin(IL)-4 both induce activation of pro-inflammatory and anti-inflammatory macrophages (M1 and M2, respectively), but he impact of saliva on programming bone marrow cells into either M1 or M2 macrophages remains unclear. Methods: Fresh sterile saliva, similar to LPS, was used in culture of mouse bone marrow to observe its affect on the process of macrophage polarisation. Evaluation was conducted using real-time gene expression analysis and immunoassays for various indicators of different macrophage types. Results: Fresh sterile saliva was associated with robust activation of the M1 phenotype, characterised by a strong increase in the genes for interleukins IL-12, IL-6 and Nitric oxide synthase-2 (NOS2) observed with real-time gene expression analysis and immunoassay. It was also associated with increased expression of IL-10, which is characteristic of the M2 phenotype, but not the proteins Ym1, FIZZ-1 and arginase. Conclusion: These preliminary in vitro results show that saliva can support the formation of pro-inflammatory macrophages. We are currently evaluating the role of TLR4 signaling with the antagonist TAK-242, and the respective downstream signaling pathways, as well as the impact of the saliva pellicle on M1 or M2 macrophage activation.