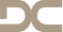-

-

Conventional sinus floor elevation with a new biphasic bone graft material and a slow-resorbing collagen membrane
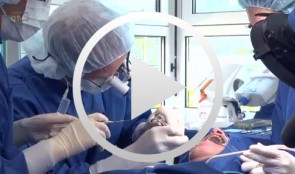
PATIENT: A 53-year-old female patient with an edentulous gap in her left molar region requested a fixed implant-supported restoration that would not require removal of the existing crowns on teeth 24 and 27. CHALLENGE: Create a sufficient amount of bone to enable implant placement in sites 25 and 26, using the layering principle. TREATMENT: Sinus-floor elevation was performed in the left maxilla, filling the bony defect with slow resorbing graft material and autogenous bone covered by a slow-resorbing collagen membrane (the layering principle). Implants were then placed in sites 25 and 26 and subsequently restored with metal-ceramic splinted crowns. -
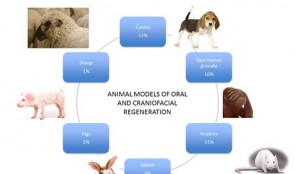

Pre-Clinical Model Development
Technological advances in engineering materials and biological agents continue to provide new potential therapies for patients whose quality of life and physical well-being are affected by severe orofacial defects, tooth loss or the consequences of gum disease. However, getting new therapies into everyday use by dental surgeons is not simple. Currently, only one in every thousand newly developed materials reaches patients, in a process that can take decades, and incurs enormous costs. To ensure that all suitable therapies developed in the laboratory become available to dentists, preclinical studies in animals must streamline neatly with clinical trials in humans, which can only happen if approval is gained by the regulatory authorities. This chapter provides guidance on the many practical issues that must be addressed before, during and after conducting animal experiments, in order to achieve the rigorous safety, efficacy and quality standards that must be met for human use. The emphasis is on effective planning and design, and choosing the most suitable animal models and techniques. The authors also outline ways to optimize and standardize experimental approaches, and set meaningful endpoints that translate directly into the clinical arena. -


Ethical Considerations for Performing Research in Animals
Laboratory tests have limited value for assessing the safety and effectiveness of new therapies in people. Tests must also be conducted on animals that resemble humans, both biologically and developmentally. We generally acknowledge that certain animals may be caught and sold, kept in captivity, or eaten, but using animals to meet human needs is as an area of huge controversy. This chapter gives a broad perspective on the ethical basis for animal experiments, drawing from the modern “common sense” view and several longstanding philosophical theories. Moral status is considered, alongside integrity, autonomy and dignity of animals, and their ability to reason, to form memories, and to experience pleasure or pain. Smaller animals, such as mice, rats and rabbits are essential for proving a basic principle or concept. Larger animals, such as goats, pigs, sheep, dogs and monkeys, are used more sparingly, not least because of the costs involved in their care; they are necessary because of their greater similarity to humans and thus are more relevant to advancing clinical practice. However, conflicts of interest tend to be larger with animals that are more similar to humans. Primate and dogs tend to evoke our compassion more strongly than rodents or animals we commonly eat. The authors provoke thought on this subject through examples in which the interests of humans are weighed up with those of animals in studies of cosmetics, childhood leukemia and dental defects. -


Goold Laboratory Practice (GLP)
All preclinical studies on therapies, procedures and materials intended for use in people must be carried out in accordance with the principles of good laboratory practice (GLP). This chapter gives an overview on the history and scope of GLP, its requirements for the submission of information, and its role in producing safe, high-quality products. Adhering to these principles can be challenging during this era of globalization, especially because different countries have their own regulatory authorities, inspection intervals, legal frameworks and administrative practices. Differences occur not only at a country level, but also at organizational, facility, staff and laboratory levels, resulting in enormous variability in the planning, monitoring, auditing, reporting and archiving of studies. This chapter outlines the responsibilities of all key players in the certification process – study directors, sponsors, principle investigators, archivists, and technical and scientific staff – and suggests improvements that support the flow, sharing and integration of study information on a global scale. A standardized approach at international, national and organizational levels will minimize barriers to information exchange and trade, and facilitate distribution of reproducible, traceable data, with a continuous line of evidence on safety and efficacy of individual technologies. -


Research Design and Biostatistical Considerations
The findings of studies are of little value without proper statistical analyses that reveal the power of the results, and separate them from events that occur simply by chance. Robust analysis also allows patterns to be observed and comparisons to be made with similar studies. However, the field of statistics can be confusing and overwhelming. This chapter provides an easily understandable summary of the key principles and parameters involved, and the aims of different statistical tests. The describes real preclinical studies on bone regeneration to illustrate concepts such as variance and skew, probability, distribution, standard deviations, and categorical and non-independent data. It also gives guidance on the importance of sample size, replication and specific methods like split-mouth designs. The main focus is on an efficient top–down approach, whereby statistical analysis starts before the study begins, and sometimes involves obtaining the advice of a qualified statistician. The role of generating explicit and biologically valid questions or hypotheses is highlighted, and the need to determine adequate sample sizes and control conditions, with suitable endpoints and collection of only relevant data. Planning the analytical approach in this way produces clinically applicable results and allows meaningful conclusions to be drawn within and across studies. As such, getting the statistics right is essential for translating laboratory findings into clinical practice. -
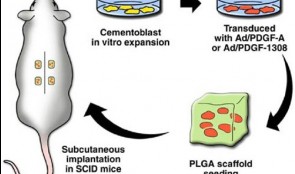

Screening Models for Tissue Engineering
These tests are carried out before preclinical studies. They are essential for exploring fundamental responses to procedures such as creating wounds in gum and bone and inserting foreign materials. Success of these interventions depends on the stability of the supporting tissues and the capacity to heal, involving the formation of blood clots and granulation tissue, and the growth of new bone and soft tissue. Screening studies tend to use small animals, often rats, to quickly and easily identify agents that show promise for preclinical studies in larger animals, which are more expensive and time consuming. The models described in this chapter include rats with induced defects of the long bones, the mandibular symphysis and ramus, and the calvarium, and addresses their suitability for testing different shapes, features and locations of implants. These studies yield information on biocompatibility at cellular, vascular and biochemical levels, as well as responses to surgical trauma and repair. The chapter emphasizes the need to use standardized, validated screening protocols to generate reproducible results, and cites specific protocols developed by various international organizations. If all screening tests meet certain basic criteria on study variables, control groups and the accessibility, homogeneity and mechanical stability of implant sites, they will comply with regulatory requirements and allow easy comparison with the existing evidence base. -

Soft Tissue Regeneration
Stable soft tissues are essential for the stability of teeth, and patients in need of restorative therapy require a solid foundation for implants. Dental surgeons can graft healthy tissue or insert scaffold material into defective areas to promote regeneration of tissues and stimulate integration. Research has recently focused on the development of new materials that are being tested in preclinical models. Two specific methods are described in this chapter to evaluate the performance of different grafts for gain of keratinized tissue and soft tissue volume. The presented models allow studying tissue integration and regeneration and serve as standardized approaches. The rich information derived this way will make it easier to predict outcomes in humans, and hasten the use of new graft materials in patients. -


Periodontal Regeneration
Animals with defects of the gum can be used to investigate the effectiveness and safety of scaffold materials, devices and biologics for bone and soft tissue repair, before they are tested in people. Living models are essential for observing changes in structure and function over time, sometimes long periods, during the remodeling and healing phases. This chapter recommends which animals are best suited for studying agents such as growth factors and barrier membranes. They include both small and large animals, such as rats, dogs and primates, with both natural defects and surgically or ligature-induced defects. The design of the studies is addressed specifically, with instructions on the creation of various standardized defects, and how to care for the animals before and after surgery, including management of biohazardous materials such as viral vectors. The roles of institutional guidelines and the requirements of regulatory bodies and animal housing authorities are also covered. Investigators who are studying the healing process sometimes need guidance on selecting suitable endpoints that can be adapted to humans in clinical settings; several measurable outcomes are specified here, based on histologic, morphometric and imaging findings, which aim to provide relevant and reproducible data on molecular and cellular responses, and entire gum tissue reactions to the intervention. -

Implant Bone Dentures with Bone Augmentation and Soft Tissue Transplantation for Papillary Regeneration (Clinic and Laboratory)
Körner, Gerd / Müterthies, KlausDrill guide preparation with the aid of a temporary; Bone augmentation using a bone block graft; Implant insertion; Augmentation of gingival margin with a soft tissue graft from the roof of the mouth; Preparation of tooth replacements according to esthetic criteria, in some cases, as all-ceramic crowns.